Last year, scientists in Mexico have used microscopic polystyrene spheres to model how atoms bond (ie 3D shapes) AND model the polymerisation process. The video below shows the particles behaviour in the modelling experiments and the article found here has a good discussion about the advantages of this type of modelling in Chemistry - ie it relates to Skill 14.1f that we are studying in this experiment.
From our modelling
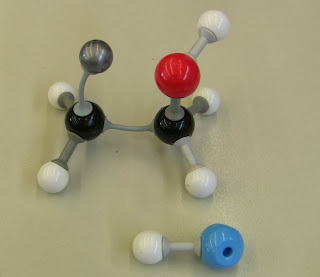
HYDRATION
From our modelling
DEHYDRATION
Worksheet Feedback
These tips should help you to correct your worksheet. Relevant number codes are written on your booklet.Q2 & Q3) The hydroxyl group has its free electron on the oxygen ato so be careful to show the bond between the carbon and hydroxyl actually connecting the C to the O, not the H.
Q4) Boiling and melting are physical changes. They do not involve chemical bonds breaking. They involve INTERMOELCULAR bonds breaking – ie the bonds between molecules. Thus substances that form very strong intermolecular bonds have higher mpt and bpt than substances that form weak intermolecular bonds. In order of strength of intermolecular bonds: Hydrogen bonds > dipole-dipole>weak dispersion. ALSO to compare alkanols and alkanes properly you need to outline the different intermolecular bonding that both can form.
Q5 & Q6)
* When talking about solubility you should always say like-dissolves-like, & say whether the solute and solvent are polar or non-polar and then say whether they are soluble or insoluble. For alkanols as the C-chain length increases the hydroxyl group becomes less significant thus the chain is less polar…
* Ethanol has a polar component (OH) and a non-polar component (the alkyl group).
Q6)
* Answer the whole question. It is OK to say why ethanol is miscible in water and hexane, but you have to explain why hexane and water are not miscible. NB molecules CAN FORM intermolecular bonds, they cannot ‘have’ or ‘contain’ them.
* There is a big difference between a hydroxide ion and a hydroxyl group. See the first page of our 9.2.3 notes.
Q7 & Q8)
* Always draw structural diagrams for carbon compounds (including addition and dehydration reactions) and always include required catalysts and states. The only reactions you DON'T need structural equations for are fermentation & combustion.
Q8)
*For alkanes, alkenes and alkynes for 1-4 carbons atoms they are gas, above that they are liquids (eventually solids but we don’t need to worry about that transition). However, because alkanols have hydrogen bonding between their molecules they are liquids (ie methanol, ethanol… are liquids)
* The naming on that sheet was very bad. 8d should have been PROPENE (there is only one isomer = no need for numbers).
* For dehydration you need a concentrated sulfuric acid catalyst, for hydration you need dilute sulfuric acid. Also note that many hydrations and dehydrations can result in two different products. Eg hydration of propene could produce propan-1-ol or propan-2-ol.
* Dehydration will only convert an alkanol to an alkene with the same number of carbons. It will not crack it into ethylene molecules. Eg pentan-1-ol will dehydrate to pent-1-ene.
* Include names remembering the correct naming has the number before the –ol or –ene NOT at the start of the name.
Summary Feedback
A) The catalyst for ethanol dehydration is CONCENTRATED sulfuric acid. Hydration of ethylene requires heating it and water in the presence of a DILUTE sulfuric acid catalyst.
B) For the explanations make sure you say why using ethylene to produce ethanol is non sustainable AND why we need renewable sources of ethylene (i.e. why we need ethylene and why making it from ethanol is sustainable). In addition - you must show how ethanol is renewable - ie it is made by fermenting ??? which comes from ??? during ???.
C) Using colours to highlight the species that change positions make it easier to see where the bonds are being broken and remade (i.e. especially the H, OH that are involved in bonding). - BUT YOU MUST USE SOME SORT OF KEY. Eg you might use a circle to indicate hydrogen, but for one that takes part in the reaction you might put a pink mark next to, the other a blue. Thus you can see exactly where these two H atoms go in the reaction.
D) You need to show all the intermediate steps to show how the catalyst reacts to make intermediate products and then reforms. Using arrows (and colours as described in C) will help make this clear.
E) You need to include TWO advantages and TWO disadvantages You must refer to BTH the Molymod and the computer animations. A big advantage of this modelling as that it enabled you to see how the catalyst played a role in the reaction! Hints for disadvantage: did it react spontaneously as in real life. Were you bale to model the individual electrons, did the models really show the correct size and position of atoms and bonds…
F) Catalysts increase reaction rate BY reacting with the reactants to produce… At the end of these intermediate steps the catalyst is…
G) It is not enough to say that using HX was simpler. You need to say WHY it was valid to simplify it as HX – you must relate it to what happened in the reaction.
H) Use structural equations & states. NB the ethanol dehydrated starts off as pure ethanol. The ethylene is a gas and being non-polar will not dissolve in the water (ie cannot be aq).
I) Hydration of ethylene does not involve cracking. However, cracking of crude oil fractions was used to produce the ethylene which was then hydrated (in an addition reaction with water) to produce ethanol.








No comments:
Post a Comment