Q1) When you are asked to compare two things in Chemistry you need to communicate the meaning (definition) of both things AND the similarities & differences between them. Considering that CFC’s are a special subset of haloalkanes it would be easier to define what a haloalkane is and then the comparative definition of the CFC. Eg “Haloalkanes are alkanes (single bonded hydrocarbon chains) that have…. However, CFCS….”
Q2) Don’t forget: you need to use di, tri, tetra etc if there are multiple of a halogen; you need to give the lowest numbers to the FIRST ALPHABETICAL halogen IF there are two equivalent numbering systems; & you need to always name the halogens in alphabetical order.
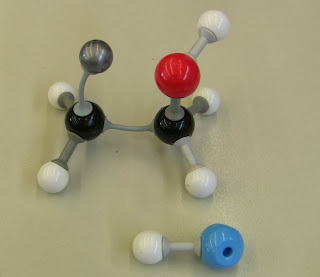
Eg for the compound below, numbering from either end gives the same set of numbers. So we give the first alphabetical halogen (chlorine) the smallest number - BUT - we still name it in alphabetical order.
So the name is...(highlight the space below to check your answer)
2-chloro-3-fluorobutane
BTW - there is currently a lot of controversy about this at the moment - the above explanation is correct according to IUPAC and RACI but this WAS NOT one of the provided answers to a MC question in the 2012 HSC Chemistry paper. Dr Blurg (& I imagine a lot of other teachers) contacted the BOS about this and they released a statement the essentially admitted their error in early 2013. Go Dr Blurg!
Q3) Yes you need to use the correct chemical symbols and include all the hydrogens.
Q4) Make sure you read the question (ie name…) and also see the tips for Q2 above.
Q5) See tips for Q2 and Q4 above.
Q6a) See (general) tips for Q1 above
Q7) The whole 9.4.4 focus area is about how Cl free radicals catalyse ozone decomposition. Thus, when it asks why HCFC’s are being phased out and why HFC’s are preferred you need to make sure that your answer clearly explains this in terms of Cl.
Summary Feedback
A) When presenting your rules you need to be thorough. Eg don’t forget to:
* Say what needs to be named alphabetically.
* Define the prefixes to use (up to five min).
* Define what is meant by ‘lowest total’ numbers.
* Specify what has to be done if there are two equivalent numbering systems - Hint it is NOT to give preference to the most electronegative halogen!
* Identify where commas and hyphens are needed.
B) In the risk assessment you need to consider why you need to pick up dropped pieces and why you should not use your mouth to separate the models.
C) Make sure you use correct naming when naming your examples and isomers. Don't make it difficult for yourself. Put all the halogens down one end so there is no confusion about the end to name from.
D) Be careful to be exact with your definitions. Eg you need to indicate that in CFC’s chlorine AND fluorine have replaced ALL hydrogen atoms (ie they contain chlorine, fluorine and carbon atoms only). Make sure your example chemical matches the definition.
E) All required structural diagrams must be submitted (hand drawn).
F) For the last question of the Theory section you needed to draw AND name THREE isomers AND the original haloalkane.
G) Isomers have to have exactly the same number of each atom in the molecule.
H) Don't forget to include a specific application of the CFC you chose.
This summary and worksheet was done very well - AND the class got its first 'full-mark' draft - impressive. Just like the comics below!
And as this is the last 'official' post before Christmas - "MERRY CHRISTMAS BLURGLINGS"
Enjoy this little game and the pics below